Proteolytic processing of neuregulin 1 type III by three intramembrane cleaving proteases
J Biol Chem. 2016 Jan 1;291(1):318-33.
| Authors/Editors: |
Fleck D Voss M Brankatschk B Giudici C Hampel H Edbauer D Steiner H Kremmer E Haug-Kröper M Rossner MJ Fluhrer R Willem M Haass C |
|---|---|
| Publication Date: | 2015 |
| Type of Publication: | Journal Article |
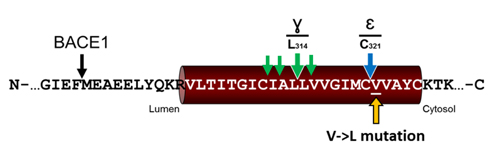
Numerous membrane-bound proteins undergo regulated intramembrane proteolysis (RIP). RIP is initiated by shedding and the remaining stubs are further processed by intramembrane cleaving proteases (I-CLiPs). Neuregulin 1 type III (NRG1 type III) is a major physiological substrate of β-secretase (β-site APP cleaving enzyme 1; BACE1). BACE1-mediated cleavage is required to allow signaling of NRG1 type III. Due to the hairpin nature of NRG1 type III two membrane-bound stubs with a type 1 and a type 2 orientation are generated by proteolytic processing. We demonstrate that these stubs are substrates for three I-CLiPs. The type 1 oriented stub is further cleaved by γ-secretase at an ε-like site 5 amino acids N-terminal to the C-terminal membrane anchor and at a γ-like site in the middle of the transmembrane domain. The ε-cleavage site is only 1 amino acid N-terminal to a V/L substitution associated with schizophrenia. The mutation reduces generation of the NRG1 type III β-peptide as well as reverses signaling. Moreover, it affects the cleavage precision of γ-secretase at the γ-site similar to certain Alzheimer's disease associated mutations within the Amyloid precursor protein. The type 2 oriented membrane-retained stub of NRG1 type III is further processed by signal peptide peptidase-like proteases SPPL2a and SPPL2b. Expression of catalytically inactive aspartate mutations as well as treatment with (Z-LL)2 ketone inhibits formation of a N-terminal ICD and the corresponding secreted C-peptide. Thus, NRG1 type III is the first protein substrate, which is not only cleaved by multiple sheddases but also processed by three different I-CLiPs.