Arginine methylation next to the PY-NLS modulates Transportin binding and nuclear import of FUS
EMBO J. 2012 Sep 11. doi: 10.1038/emboj.2012.261. [Epub ahead of print]
| Authors/Editors: |
Dormann D
Madl T
Valori CF
Bentmann E
Kremmer E
Ansorge O
Mackenzie IR
Neumann M
Haass C
|
| Publication Date: |
2012 |
| Type of Publication: |
Journal Article |
ABSTRACT:
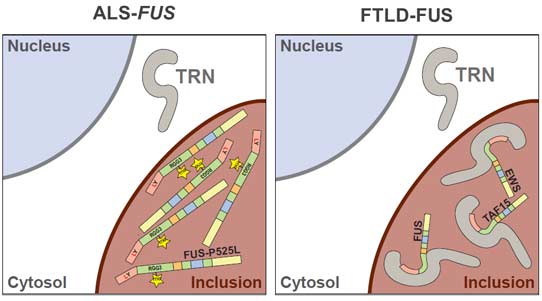
Fused in sarcoma (FUS) is a nuclear protein that carries a proline-tyrosine nuclear localization signal (PY-NLS) and is imported into the nucleus via Transportin (TRN). Defects in nuclear import of FUS have been implicated in neurodegeneration, since mutations in the PY-NLS of FUS cause amyotrophic lateral sclerosis (ALS). Moreover, FUS is deposited in the cytosol in a subset of frontotemporal lobar degeneration (FTLD) patients. Here, we show that arginine methylation modulates nuclear import of FUS via a novel TRN-binding epitope. Chemical or genetic inhibition of arginine methylation restores TRN-mediated nuclear import of ALS-associated FUS mutants. The unmethylated arginine-glycine-glycine domain preceding the PY-NLS interacts with TRN and arginine methylation in this domain reduces TRN binding. Inclusions in ALS-FUS patients contain methylated FUS, while inclusions in FTLD-FUS patients are not methylated. Together with recent findings that FUS co-aggregates with two related proteins of the FET family and TRN in FTLD-FUS but not in ALS-FUS, our study provides evidence that these two diseases may be initiated by distinct pathomechanisms and implicates alterations in arginine methylation in pathogenesis.
Related Links




