Requirements for Stress Granule Recruitment of Fused in Sarcoma (FUS) and TAR DNA Binding Protein of 43kDa (TDP-43)
J. Biol. Chem. 2012 May 4. [First Published]
| Authors/Editors: |
Bentmann E Neumann E Tahirovic S Rodde R Dormann D Haass C |
|---|---|
| Publication Date: | 2012 |
| Type of Publication: | Journal Article |
ABSTRACT:
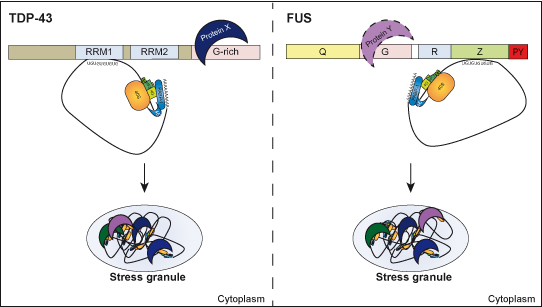
Cytoplasmic inclusions containing TAR DNA binding protein of 43 kDa (TDP-43) or Fused in sarcoma (FUS) are a hallmark of amyotrophic lateral sclerosis (ALS) and several subtypes of frontotemporal lobar degeneration (FTLD). FUS-positive inclusions in FTLD and ALS patients are consistently co-labeled with stress granule (SG) marker proteins. Whether TDP-43 inclusions contain SG markers is currently still debated. We determined the requirements for SG recruitment of FUS and TDP-43 and found that cytoplasmic mislocalization is a common prerequisite for SG recruitment of FUS and TDP-43. For FUS, the arginine-glycine-glycine zinc finger domain, which is the protein´s main RNA binding domain, is most important for SG recruitment, while the glycine-rich domain and RNA recognition motif (RRM) domain have a minor contribution and the glutamine-rich domain is dispensable. For TDP-43, both the RRM1 and the C-terminal glycine-rich domain are required for SG localization. ALS-associated point mutations located in the glycine-rich domain of TDP-43 do not affect SG recruitment. Interestingly, a 25 kDa C-terminal fragment (CTF) of TDP-43, which is enriched in FTLD/ALS cortical inclusions but not spinal cord inclusions, fails to be recruited into SG. Consistently, inclusions in the cortex of FTLD patients, which are enriched for CTFs, are not co-labeled with the SG marker Poly A binding protein 1 (PABP-1), whereas inclusions in spinal cord, which contain full-length TDP-43, are frequently positive for this marker protein.