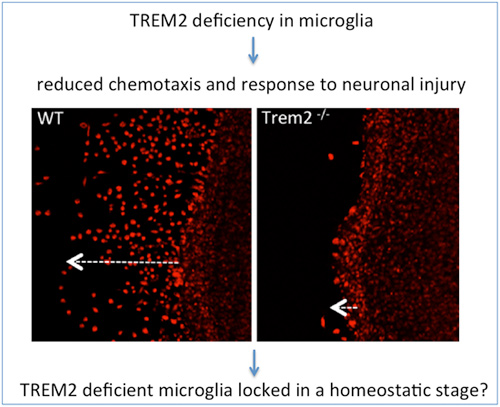
TREM2 deficiency impairs chemotaxis and microglial responses to neuronal injury
EMBO Rep. 2017 Jul;18(7):1186-1198
| Authors/Editors: |
Fargol Mazaheri Nicolas Snaidero Gernot Kleinberger Charlotte Madore Anna Daria Georg Werner Susanne Krasemann Anja Capell Dietrich Trümbach Wolfgang Wurst Bettina Brunner Sebastian Bultmann Sabina Tahirovic Martin Kerschensteiner Thomas Misgeld Oleg Butovsky Christian Haass |
|---|---|
| Publication Date: | 2017 |
| Type of Publication: | Journal Article |
Sequence variations in the triggering receptor expressed on myeloid cells 2 (TREM2) are linked to an increased risk for several neurodegenerative disorders. Trans-criptomic and functional studies reveal that TREM2 deficient microglia display a homeostatic mRNA signature and are impaired in chemotaxis and their response to neuronal injury.
* TREM2 deficiency in microglia affects expression profiles of genes clusters involved in chemotaxis.
* TREM2 deficiency impairs migration, chemotaxis and process outgrowth.
* TREM2 deficient microglia display a homeostatic mRNA signature.
Sequence variations in the triggering receptor expressed on myeloid cells 2 (TREM2) have been linked to an increased risk for neurodegenerative disorders such as Alzheimer's disease and frontotemporal lobar degeneration. In the brain, TREM2 is predominantly expressed in microglia. Several disease-associated TREM2 variants result in a loss of function by reducing microglial phagocytosis, impairing lipid sensing, preventing binding of lipoproteins and affecting shielding of amyloid plaques. We here investigate the consequences of TREM2 loss of function on the microglia transcriptome. Among the differentially expressed messenger RNAs in wild-type and Trem2-/- microglia, gene clusters are identified which represent gene functions in chemotaxis, migration and mobility. Functional analyses confirm that loss of TREM2 impairs appropriate microglial responses to injury and signals that normally evoke chemotaxis on multiple levels. In an ex vivo organotypic brain slice assay, absence of TREM2 reduces the distance migrated by microglia. Moreover, migration towards defined chemo-attractants is reduced upon ablation of TREM2 and can be rescued by TREM2 re-expression. In vivo, microglia lacking TREM2 migrate less towards injected apoptotic neurons, and outgrowth of microglial processes towards sites of laser-induced focal CNS damage in the somatosensory cortex is slowed. The apparent lack of chemotactic stimulation upon depletion of TREM2 is consistent with a stable expression profile of genes characterizing the homoeostatic signature of microglia.