Content
Research Focus
Aggregation and accumulation of proteins in the brain are common features of diverse age-related neurodegenerative diseases. Each of these neurodegenerative diseases is associated with abnormalities in the folding of a different protein leading to protein aggregation and ultimately to neuronal death.
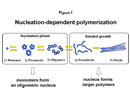
Alzheimers Disease (AD) is one of these protein conformational diseases which is characterized by the extracellular accumulation of amyloid-β peptide and neuroanatomical changes. Kinetic studies have shown that Aβ protein aggregation occur via a nucleation mechanism, which resembles a crystallization process (Figure 1). However, little is known about how protein aggregation and deposition is initiated in vivo.
We use different transgenic mouse models which develop several lesions similar to those seen in AD. Specifically we are interested in the question of how Aβ protein aggregates and forms plaques in vivo and what might trigger the initiation of plaque formation along with the pathology that accompanies it. Furthermore, we investigate the mechanisms underlying the neuritic changes and test the idea that they might have the potential to recover after treatments.
The methods we use in the lab range from biochemistry, molecular biology, stereotaxic approaches and microscopy (including 2-Photon microscopy, Figure 2) to address fundamental questions in AD research.