Content
Steiner Lab
Biochemistry of γ-Secretase
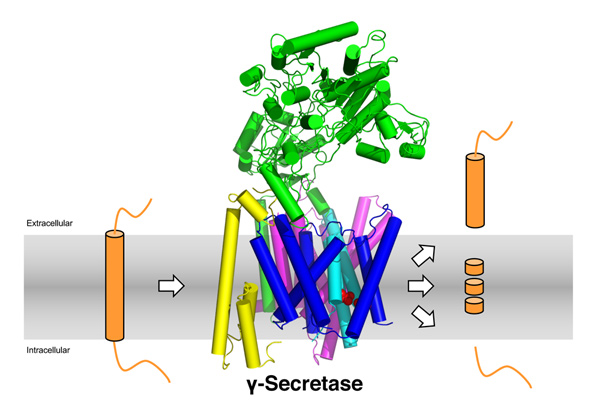
Our lab studies the function and mechanism of γ-secretase, a pivotal intramembrane protease complex critically involved in the generation of neurotoxic Alzheimer´s disease-associated amyloid-β peptides (Aβ), nuclear signaling and membrane proteostasis. We use state of the art biochemical, molecular biology and cell biological methods to get insight into how this fascinating proteolytic enzyme works.
Currently, we are focusing on how γ-secretase interacts with its substrates, how the enzyme is influenced by the membrane environment, the mechanism of how its activity is affected by familial Alzheimer´s disease mutations, and how Aβ generation can be pharmacologically modulated in a beneficial way.
Responsible for content: Prof. Dr. Harald Steiner

