The C9orf72 GGGGCC Repeat Is Translated into Aggregating Dipeptide-Repeat Proteins in FTLD/ALS
Science. 2013 Feb 7. [Epub ahead of print]
| Authors/Editors: |
Mori K Weng SM Arzberger T Rentzsch K Kremmer E Schmid B Kretzschmar HA Cruts M Van Broeckhoven C Haass C Edbauer D |
|---|---|
| Publication Date: | 2013 |
| Type of Publication: | Journal Article |
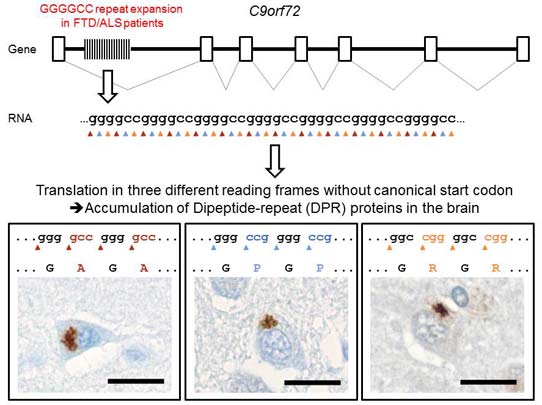
ABSTRACT:
Expansion of a GGGGCC hexanucleotide repeat upstream of the C9orf72 coding region is the most common cause of familial frontotemporal lobar degeneration and amyotrophic lateral sclerosis (FTLD/ALS), but the pathomechanisms involved are unknown. Like in other FTLD/ALS variants, characteristic intracellular inclusions of misfolded proteins define C9orf72 pathology, but the core proteins of the majority of inclusions are still unknown. Here we found that most of these characteristic inclusions contain poly-(Gly-Ala) and to a lesser extent poly-(Gly-Pro) and poly-(Gly-Arg) dipeptide-repeat proteins presumably generated by non-ATG-initiated translation from the expanded GGGGCC repeat in three reading frames. These findings directly link the FTLD/ALS-associated genetic mutation to the predominant pathology in patients with C9orf72 hexanucleotide expansion.



