The heat shock response is modulated by and interferes with toxic effects of scrapie prion protein and amyloid β
J Biol Chem. 2012 Dec 21;287(52):43765-76. doi: 10.1074/jbc.M112.389007. Epub 2012 Oct 31
| Authors/Editors: |
Resenberger UK
Mueller V
Munter LM
Baier M
Multhaup G
Wilson MR
Winklhofer KF
|
| Publication Date: |
2012 |
| Type of Publication: |
Journal Article |
ABSTRACT:
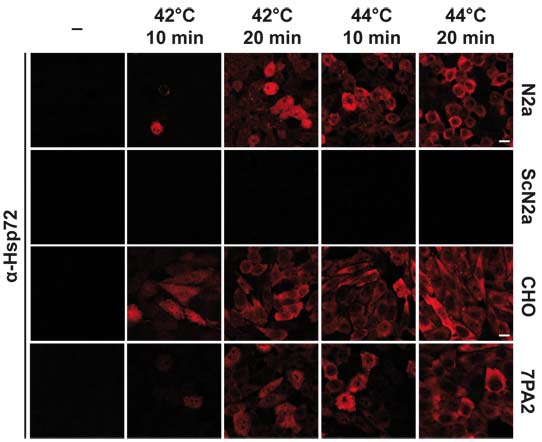
The heat shock response (HSR) is an evolutionarily conserved pathway designed to maintain proteostasis and to ameliorate toxic effects of aberrant protein folding. We have studied the modulation of the HSR by the scrapie prion protein (PrPSc) and amyloid beta peptide (Aβ) and investigated whether an activated HSR or the ectopic expression of individual chaperones can interfere with PrPSc- or Aβ-induced toxicity. First, we observed different effects on the HSR under acute or chronic exposure of cells to PrPSc or Aβ. In chronically exposed cells the threshold to mount a stress response was significantly increased, evidenced by a decreased expression of Hsp72 after stress, while an acute exposure lowered the threshold for stress-induced expression of Hsp72. Next, we employed models of PrPSc- and Aβ-induced toxicity to demonstrate that the induction of the HSR ameliorates the toxic effects of both PrPSc and Aβ. Similarly, the ectopic expression of cytosolic Hsp72 or the extracellular chaperone clusterin protected against PrPSc- or Aβ-induced toxicity. However, toxic signaling induced by a pathogenic PrP mutant located at the plasma membrane was prevented by an activated HSR or Hsp72 but not by clusterin, indicating a distinct mode of action of this extracellular chaperone. Our study supports the notion that different pathological protein conformers mediate toxic effects via similar cellular pathways and emphasizes the possibility to exploit the heat shock response therapeutically.
Related Links




